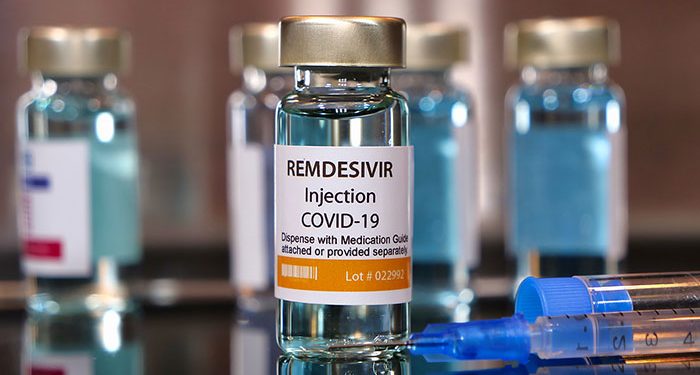Antiviral drug Remdesivir, sold under brand name Veklury, has become the first drug to get FDA’s authorization for the treatment of the novel coronavirus. The drug has been approved for the treatment of adults and children above the age of 12 and weighing 88 pounds (40kg) or more., According to the FDA, Remdesivir is to be used for COVID-19 patients who require hospitalization and should be used in medical centers which can provide comprehensive care and monitoring of patients.
The approval is coming months after Remdesivir was initially approved for all COVID-19 patients on May 1, 2020, under an Emergency Use Authorization (EUA). There are also ongoing trials to ascertain how safe and effective Remdesivir is in treating COVID-19 patients that are less than 12 years old and weigh at least 3.5kg.
“The FDA is committed to expediting the development and availability of COVID-19 treatments during this unprecedented public health emergency,” Commissioner of the administration, Stephen M. Hahn, stated. “Before granting approval, the FDA critically evaluated the results of different clinical trials. As part of our Coronavirus Treatment Acceleration Program, we will ensure that new medical products get to patients early and we will keep testing for possible risks and effectiveness in the products.”
The Federal Food, Drug, and Cosmetic Act stipulate that a new drug or other medical product can only be authorized for public use after adequate evidence points to its effectiveness and safety. The Act mandates the FDA to assess the benefits and risks of the product and only give approval if the benefits substantially outweigh the risks, though this does not apply to a EUA. A similar benefit-risk assessment was carried out on Veklury using data from three unrelated clinical trials with participation from people in different age brackets with mild to severe cases of COVID-19.
The three clinical trials checked for the number of days it took patients using Veklury to recover, a possible reduction in the severity of symptoms, and a drop in the mortality rate, and some other changes in patients using Veklury. All three trials helped the FDA conclude its assessment of Veklury and led to its approval.
The approval also comes with essential information such as the required dosage, possible side effects, and contra-indication of Veklury. Accelerated levels of liver enzymes, drop in blood pressure, levels of oxygen in the blood, shortness of breath, nausea, shivering, and swelling are some of the side effects of the antiviral drug.
Other safety information about the administration of the drug on COVID-19 patients is available in the fact sheets for patients, medical practitioners, and other caregivers. The administration is charged with protecting the health of Americans by ensuring that a medical product manufactured for humans or animals is safe, effective, and comes with minimal risks. It also ensures that food, cosmetics, supplements, medical devices, and others are safe and effective.
Source: fda.gov